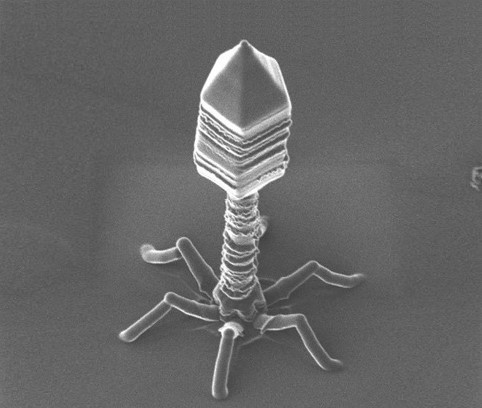
The development of bacteriophage resistance in Vibrio alginolyticus depends on a complex metabolic adaptation strategy
Marine Bacteriophages
Dr. Emmanouil Flemetakis, Associate Professor
Dr. Dimitrios Skliros, Post-doctoral researcher
Laboratory of Molecular Biology, Department of Biotechnology, School of Applied Biology and Biotechnology, Agricultural University of Athens
Multi-drug resistance of fish pathogenic bacteria is being described in fish hatcheries over the last years. Researches have been reporting a promising alternative solution, that of using bacteriophages against them, in order to reduce antibiotics usage. To this extend, earlier this year Skliros et al. from the Agricultural University of Athens, published an article in the Viruses journal, which dives in and explores the biochemical traits that the aquatic fish pathogenic bacterium Vibrio alginolyticus acquire after interaction with its natural viral predators, namely bacteriophages. But what are bacteriophages anyway?
Bacteriophages or Phages are the most abundant entity in the (micro-) world. They are viruses infecting explicitly bacteria. Their numbers are estimated ten times more than bacteria and are held accountable for the lysis of 40% of bacterial cells in the ocean every day. Although their lifecycle reminds that of a virus with an infection process, including a dormant period and host lysis after multiplication, still their looks are quite alien. Sea is a habitat that assists frequent interactions between bacteriophages and host bacteria, but only recently researchers have begun to understand that this relationship is far apart from a simple binary model between a hunter and prey. It is now evident that bacteriophages can drive bacteria phenotypic traits or even accelerate the evolution dynamics of their hosts, as the latter tries to become phage tolerant. This tolerance or resistance comes either through genomic mutations or metabolic adaptation strategies which result to a nutrient or fitness cost.
DID YOU KNOW?
Researchers have been attempting to exploit the lytic nature of bacteriophages against pathogenic bacteria since 1920s. Nowadays there is an organized effort against multi-drug resistant bacteria with the use of bacteriophages. This application is being named as “phage therapy“.
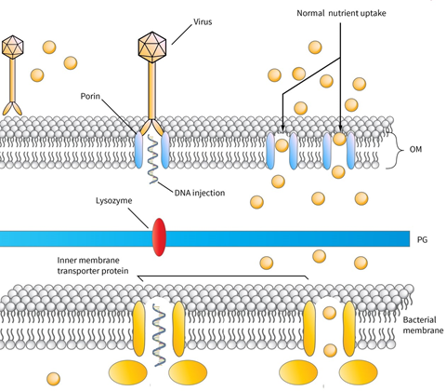
The infection process of lytic bacteriophages in Gram-negative bacteria
In this picture, the infection process of a virus in a Gram-negative bacterium is represented. The virus is attached to a membrane porin-protein, injects its DNA in the periplasmic space, whereas a lysozyme molecule attached to phage-tail is able to degrade the peptidoglycan layer of periplasmic space. Then the phage-DNA is able to pass through naturally occurring inner-membrane proteins and start its replication in the cytoplasm. Skliros et al. demonstrated that aquatic species are able to diminish the expression of genes involved in these natural channels and achieve phage denial.
Revenge of the phages: Although this work highlights the response of bacteria against lytic bacteriophages, studies has shown that bacteriophages also evolve and find alternative roots to infect their “prey”.
In their work, Skliros et al., highlight the metabolic reprogramming the cells experience due to phage-host interactions. Moreover, by using multi-omics approaches it is evident that bacteria diminish the expression of genes related to membrane proteins, such as phage-adsorption sites and inner membrane transporter proteins, which normally serve as natural channels for nutrient uptake. This expression pattern and metabolic shift appear a la carte for every bacteriophage genera the bacteria stumble across. This metabolic reprogramming is accompanied by a general metabolic shift, but also genomic mutations. Interestingly these mutations appear in transcriptional regulators of major metabolic pathways. It is difficult to establish if these mutations govern the whole metabolic adaptation strategy of the bacteria or if their presence reflects a complimentary or a collateral effect. Nevertheless, we are now aware that aquatic Vibrio species, own complex metabolic adaptation strategies to withstand biotic stresses, such as bacteriophages, as they also do against abiotic environmental challenges. This fluidity of their metabolism could impact phenotypic traits such as growth, nutrient assimilation, motility, or even virulence. Future work will focus on bacterial responses during phage therapy protocols, and how the information about phage-presence is being transferred, through quorum sensing intercellular communication peptides.
Full article you can find here
This work received funds from the Operational Program Fisheries and Maritime 2014–2020 in the framework of the project “Biological control of pathogens in fish hatcheries using bacteriophages” 5010932.